🚀 Check out this must-read post from Business News 📖
📂 **Category**:
📌 **What You’ll Learn**:
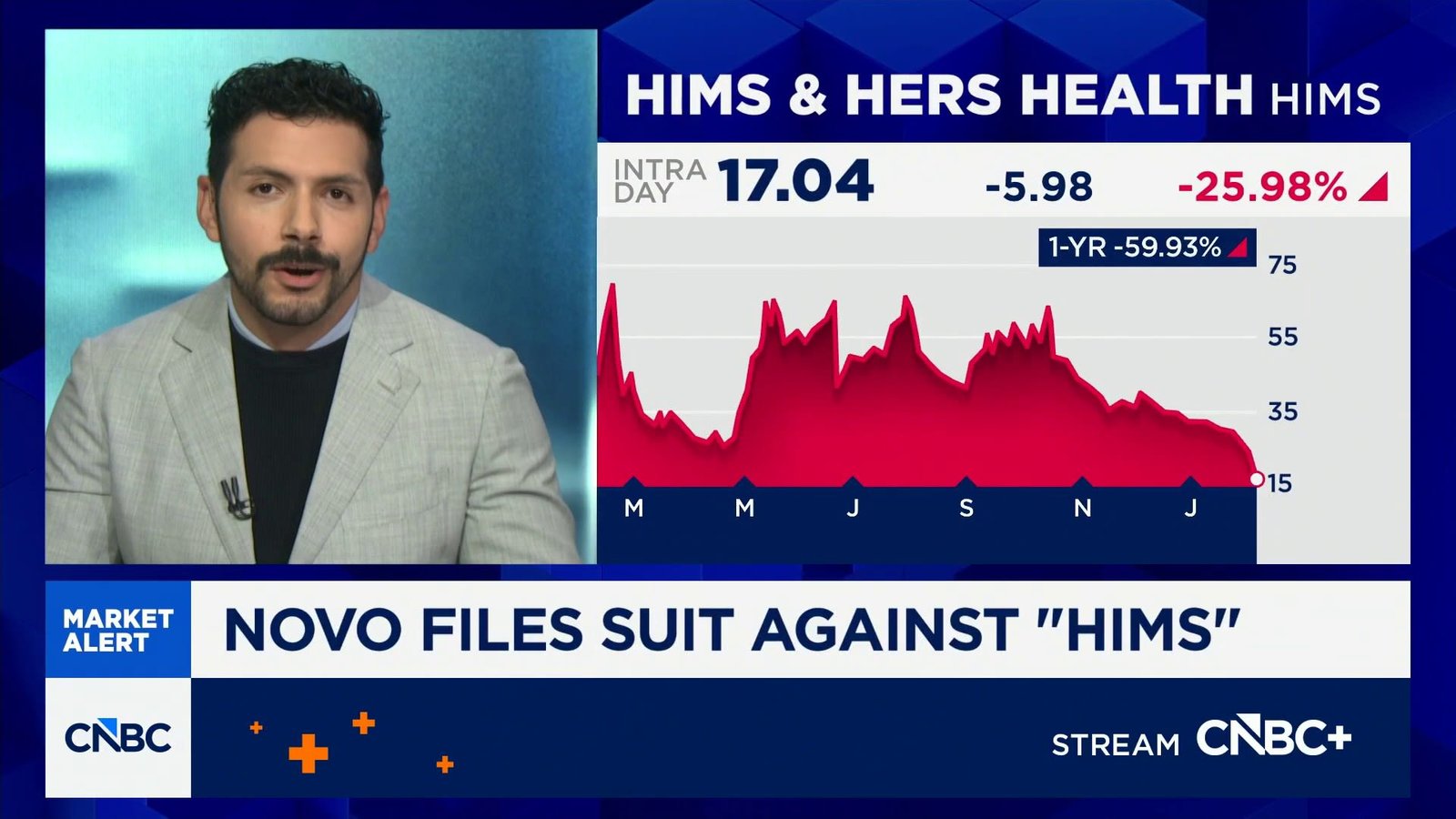
Novo Nordisk It said Monday it is filing a lawsuit against the online telehealth provider He whispered and hers To mass market cheaper, unapproved versions of the new obesity pill and injection Wegovy produced by the US pharmaceutical company
Novo is asking the court to permanently bar Hims from selling compounded versions of its drugs that infringe the company’s patents and is seeking damages.
“This is a complete hoax, and it’s been a hoax ever since the shortage ended,” John Kockelman, Novo Group’s general counsel for global legal, intellectual property and security, said in an interview.
He added: “The truth is that their medicines have not been tested, and they are putting patients at risk,” referring to the lack of verification of the safety, effectiveness and quality of compounded medicines by US regulatory bodies.
The move escalates a dispute between Novo and Hims, which said on Saturday it would stop offering the new counterfeit obesity pill after facing scrutiny from federal regulators and legal threats from the Danish drugmaker. Himes had planned to offer the drug orally for as little as $49 for the first month, about $100 less than Novo’s approved Wegovy pill.
In a statement Monday, Himes said the lawsuit is “a blatant attack by a Danish company on the millions of Americans who rely on compounded medications to access personal care” and is another case of Big Pharma “using the American judicial system as a weapon to limit consumer choice.”
Himes added that it has a “long history of providing safe access to personalized health care” for patients.
Copenhagen-listed Novo Nordisk shares were up more than 5% while NYSE-listed Hims shares were down 21% in pre-market as of 8 a.m. ET.
The lawsuit comes as Novo works to regain market share in the booming obesity drug market and fend off competition from both. Eli Lilly And a wave of complex alternatives. These copycats have proliferated under a regulatory loophole that allows companies like Hims to sell compounded versions of patented drugs when branded treatments are in short supply.
Semaglutide — the active ingredient in Novo’s popular pills and injections — is no longer in short supply in the United States, thanks to the company’s efforts to increase manufacturing capacity. No shortages have been reported for Wegovy pills, which have seen a massive launch since entering the US market in early January.
However, Novo estimated in January that as many as 1.5 million Americans use combination GLP-1 drugs.
Hims said its combination tablets and other GLP-1 products contain semaglutide, although the ingredient is protected by U.S. patents until 2032. Hims said its versions are legal because they are “tailored” in dosage.
But Novo said it does not sell semaglutide to counterfeiters directly or indirectly, and accused Himes of engaging in illegal mass formulations.
“I would just say we want to put an end to illegal mass installation,” Kockelman said, noting that Novo is not trying to stop all mass installation practices.
Compounding should be based on legitimate reasons, he said, “rather than producing a huge stock of what you call a personalized drug, which is really just a variation in dosage.”
Compounded medications may be produced on a case-by-case basis when a doctor determines they are medically necessary for a patient, such as when he or she cannot swallow a pill or is allergic to a particular ingredient in a brand-name drug.
The Food and Drug Administration announced Friday that it plans to take legal action against Hemis over the pill, including restricting access to the ingredients and referring the company to the Department of Justice over potential violations.
Some telehealth platforms, like Ro, are “doing the right things” by moving to provide patients with real, FDA-approved products from Novo and its competitors, Kockelman said.
“But some won’t, and it seems the only way we’re going to get Himes and others to stop this is through government enforcement actions and through lawsuits like the one we filed today,” he added.
Novo and Lilly have been cracking down on compounding pharmacies over the past two years, capitalizing on the growing popularity of weight loss and diabetes drugs. So far, Novo has filed about 130 lawsuits related to deceptive marketing practices and consumer fraud, Kockelman said.
Lilly went through a similar legal action with tirzepatide, the active ingredient in the weight-loss and diabetes drug Zibbond Mongaro, which is no longer available in the United States.
⚡ **What’s your take?**
Share your thoughts in the comments below!
#️⃣ **#Novo #Nordisk #sues #Hims #compounded #obesity #drugs**
🕒 **Posted on**: 1770649729
🌟 **Want more?** Click here for more info! 🌟

